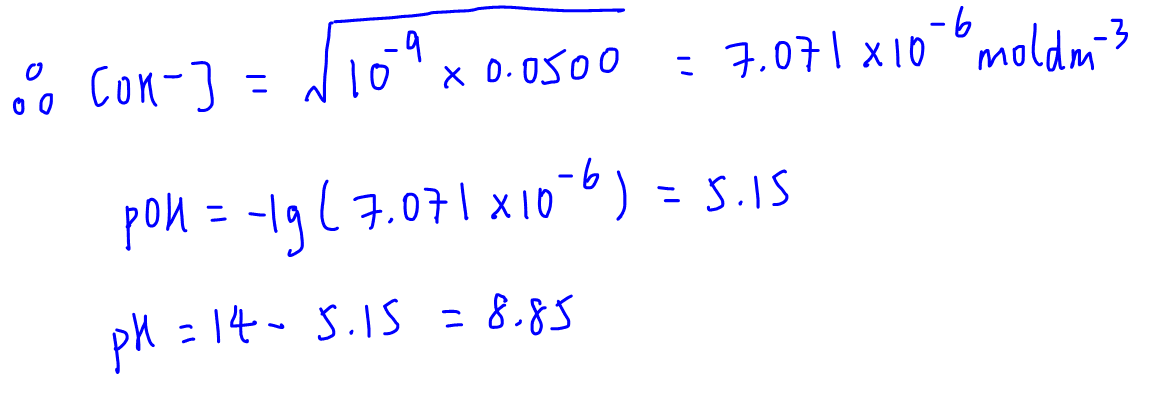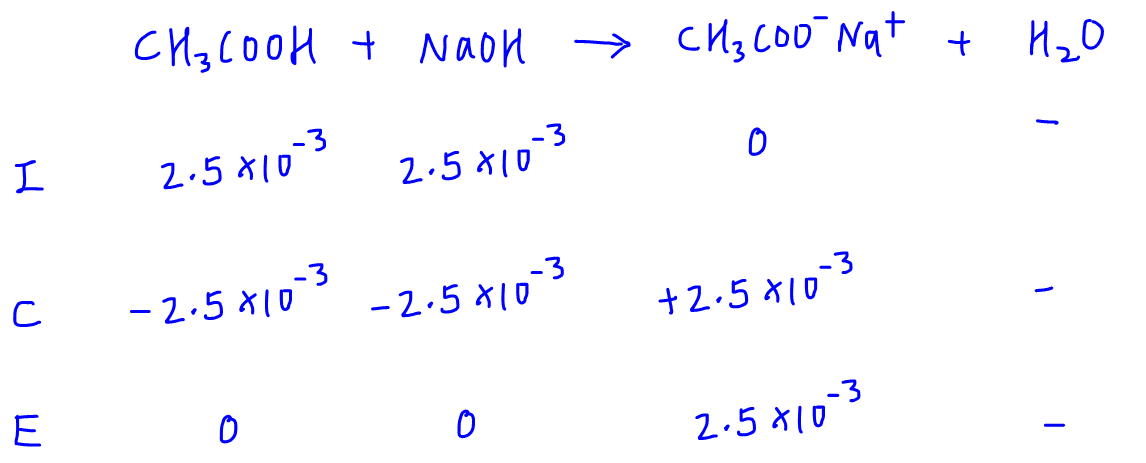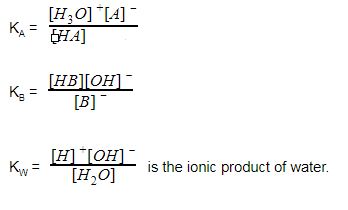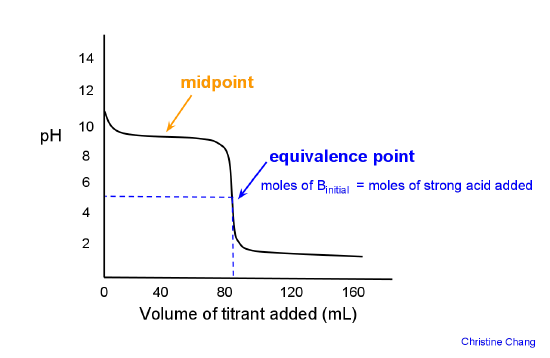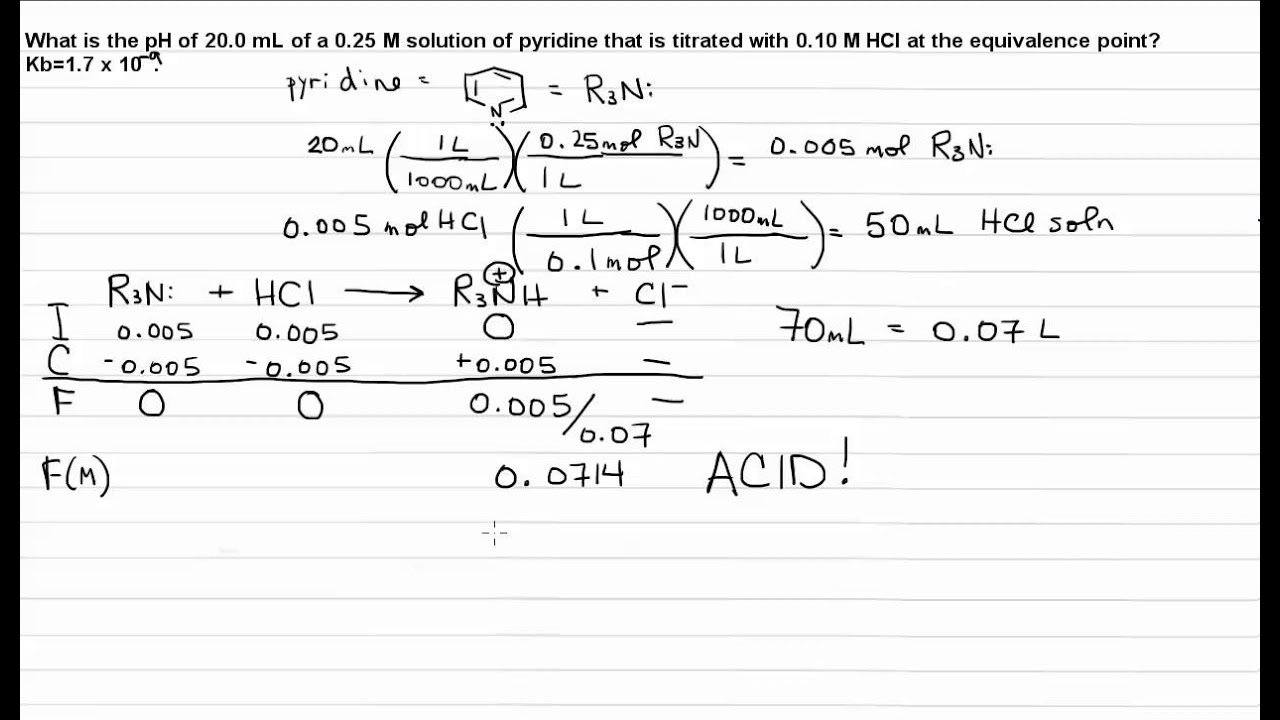
The "pH" at one-half the equivalence point in an acid-base titration was found to be 5.67. What is the value of K_a for this unknown acid? | Socratic

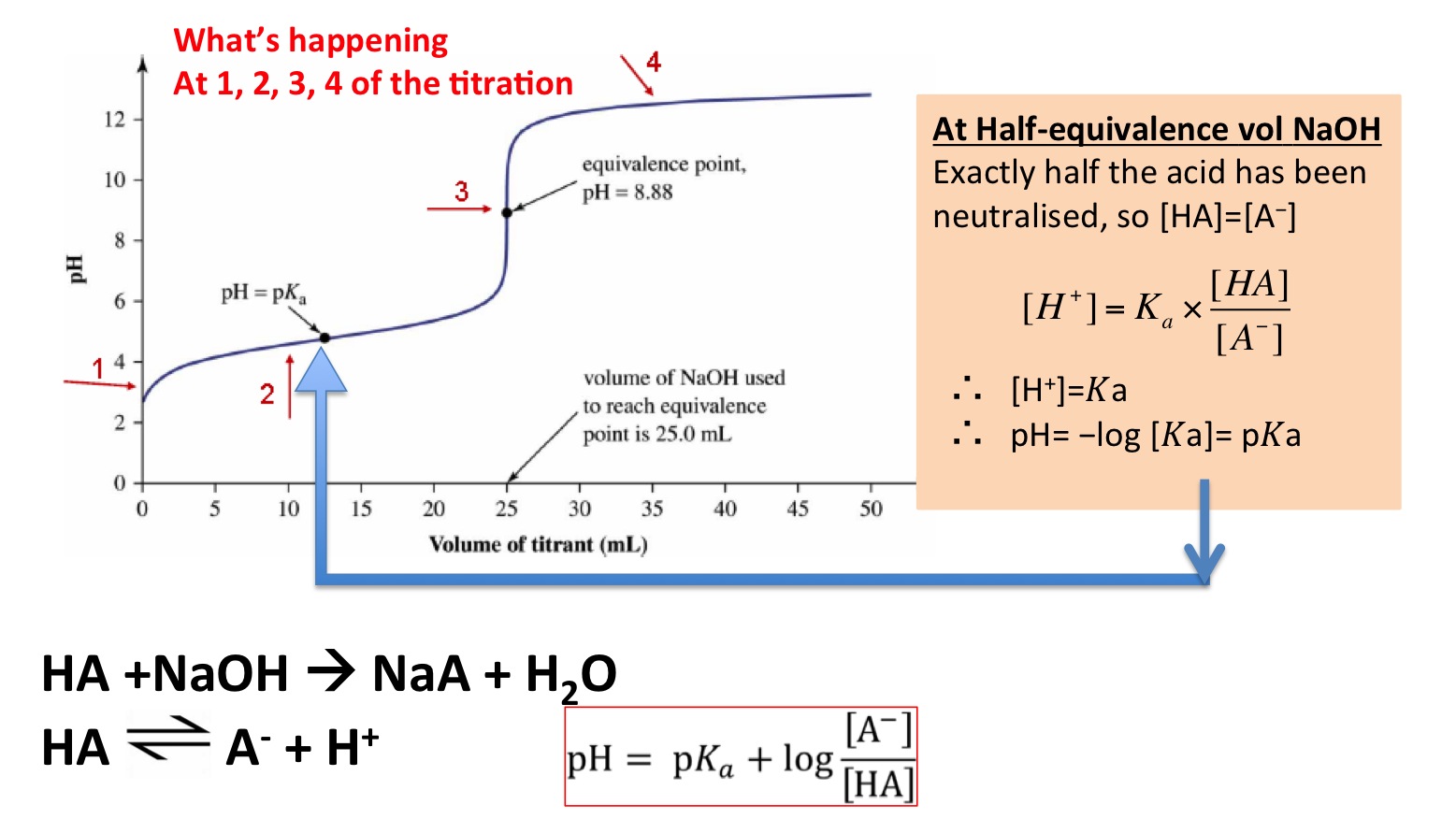
How to Calculate pKa From the Half Equivalence Point in a Weak Acid-Weak Base Titration | Chemistry | Study.com
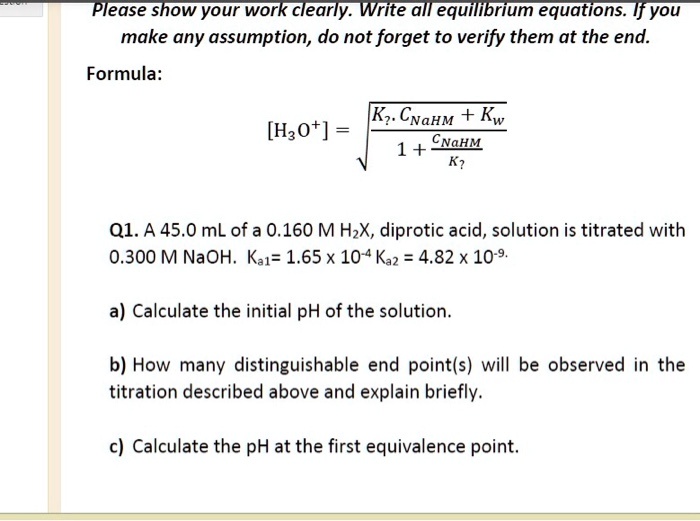
SOLVED: Please show your work clearly. Write all equillbrium equations. If you make any assumption, do not forget to verify them at the end: Formula: Kz CNaHM + Kw 1 + CNahm [
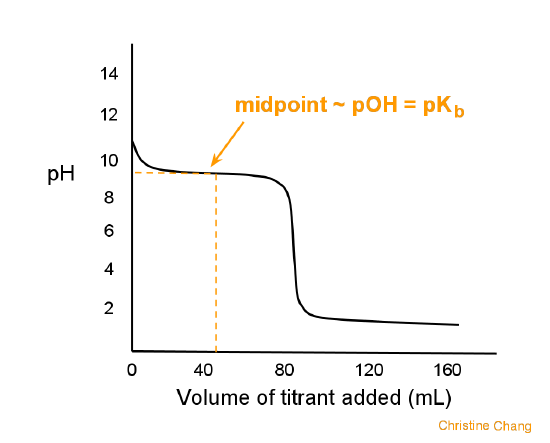
What is the difference between a half equivalence point and an equivalence point in chemistry? - Quora
Calculate the pH at the equivalence point of the titration between 0.1M CH3COOH ( 25 ml) with 0.05 M NaOH. - Sarthaks eConnect | Largest Online Education Community
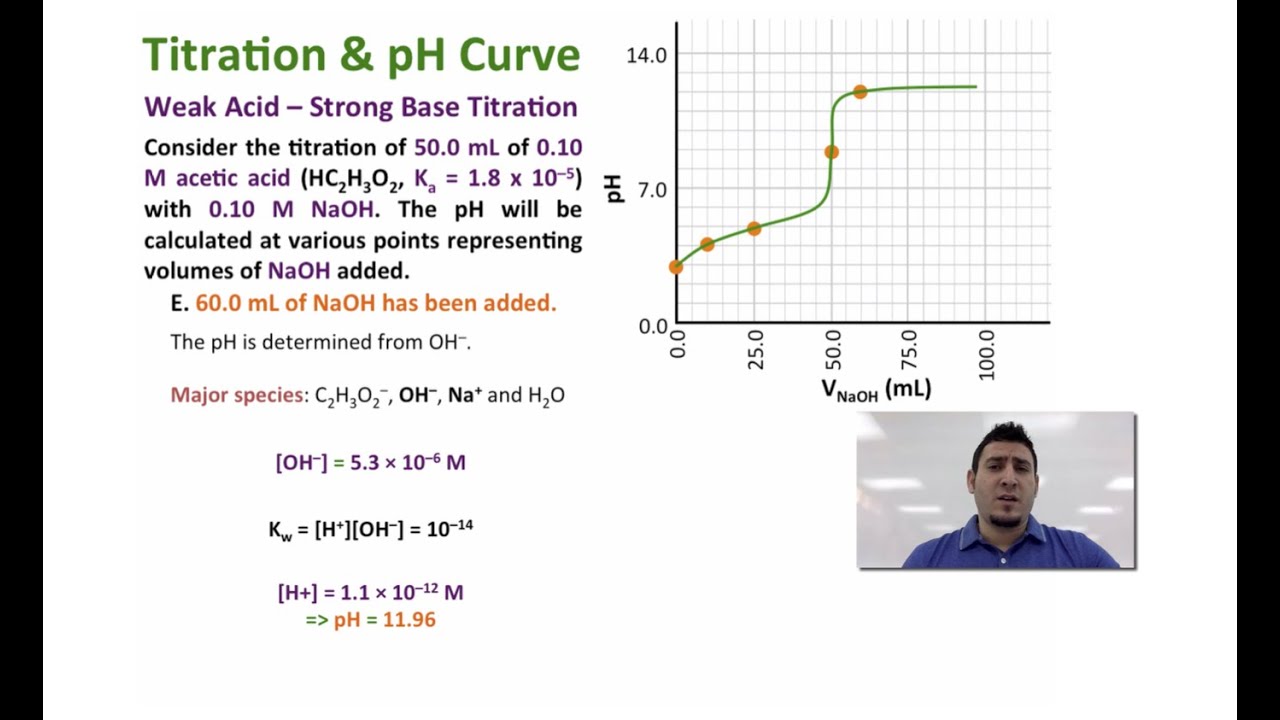
How do you calculate the pH at the equivalence point for the titration of .190M methylamine with .190M HCl? The Kb of methylamine is 5.0x10^-4. | Socratic
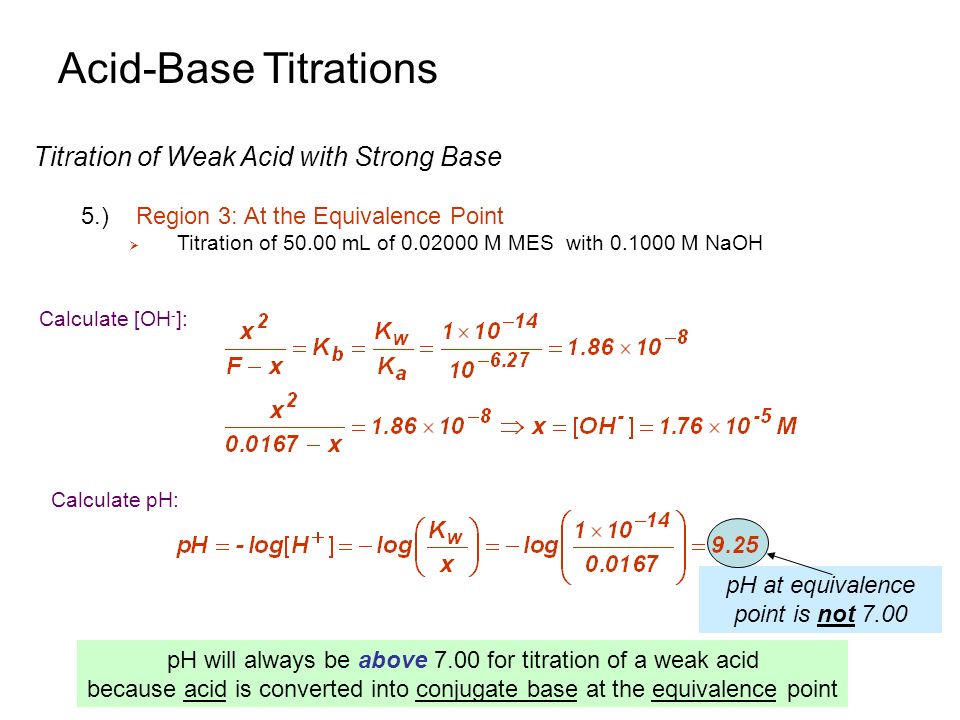
Acid-Base Titrations Introduction 3.)Overview Titrations are Important tools in providing quantitative and qualitative data for a sample. To best understand. - ppt download
![Calculate the pH at the equivalence point during the titration of 0.1M, 25 mL CH(3)COOH with 0.05M NaOH solution. [K(a)(CH(3)COOH) = 1.8 xx 10^(-5)] Calculate the pH at the equivalence point during the titration of 0.1M, 25 mL CH(3)COOH with 0.05M NaOH solution. [K(a)(CH(3)COOH) = 1.8 xx 10^(-5)]](https://d10lpgp6xz60nq.cloudfront.net/web-thumb/328699097_web.png)
Calculate the pH at the equivalence point during the titration of 0.1M, 25 mL CH(3)COOH with 0.05M NaOH solution. [K(a)(CH(3)COOH) = 1.8 xx 10^(-5)]

How to Calculate Analyte Concentration Using the Equivalence Point in an Acid-base Titration | Chemistry | Study.com

Acid/Base Titrations. Titrations Titration Curve – always calculate equivalent point first Strong Acid/Strong Base Regions that require “different” calculations. - ppt download